
A Complete Account of EMA Approvals in 2023
Shots:
-
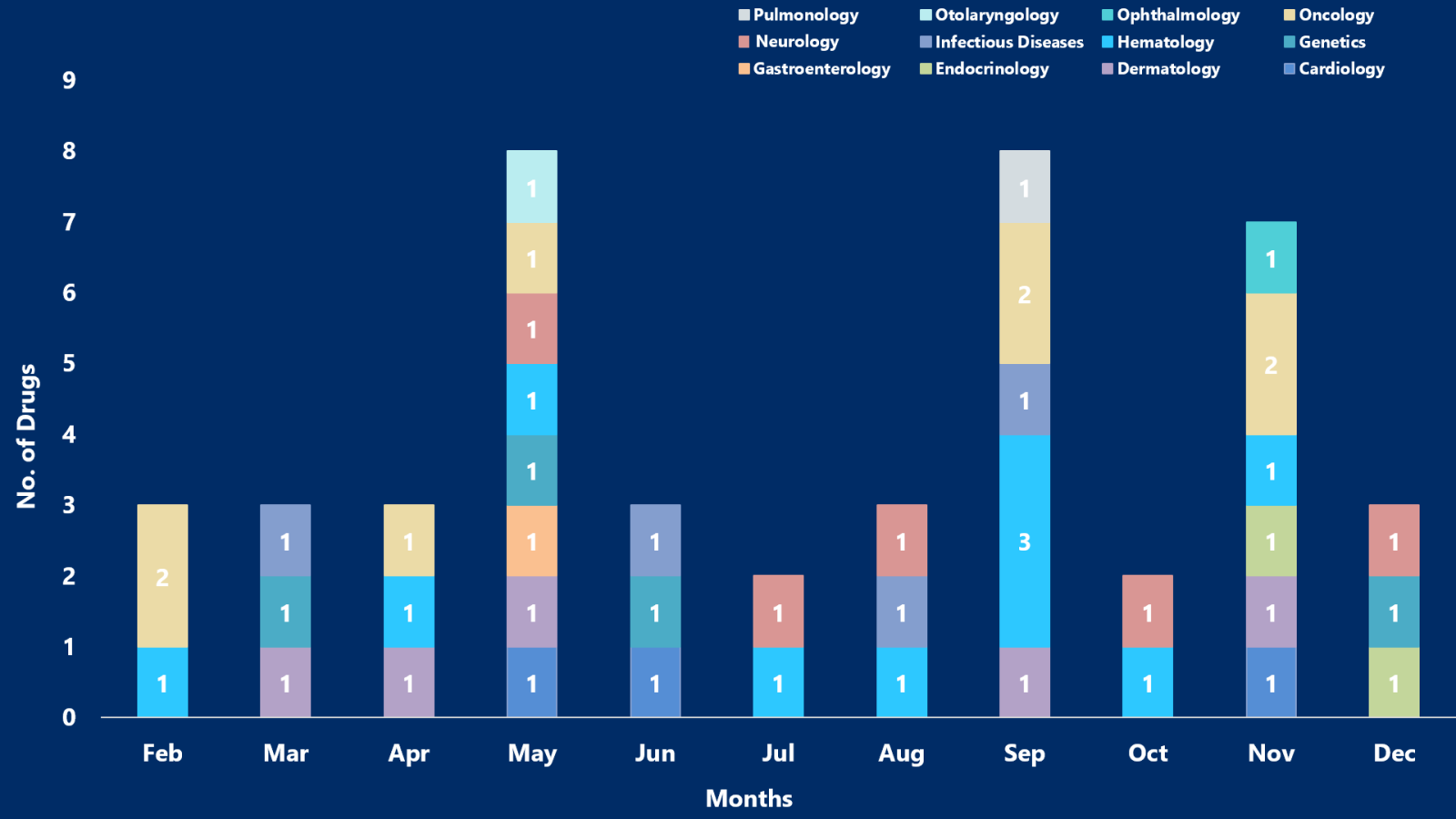
In 2023, EMA approved around 45 drugs in various therapy areas, ranging from cardiology, oncology, hematology, neurology, and dermatology to infectious diseases, genetics, pulmonary, otolaryngology, gastroenterology, ophthalmology, and endocrinology
-
PharmaShots, in an illuminating report, brings a condensed analysis of the approved drugs with the most explored areas being Oncology, Hematology, Neurology, & Dermatology
-
For the complete report with analysis, reach out to us at connect@pharmashots.com
Unveiling a second package of delights that is sure to take you down memory lane with PharmaShots EMA's new drug approvals in 2023. The report outlines the therapy area of approval, the company involved, and the approval date.
Oncology
After the USA, Europe remains the second region to conduct studies on oncology. 2023 witnessed eight noteworthy approvals. Stemline’s Orserdu has been approved as a monotherapy for the treatment of postmenopausal women, and men, with HER2-negative, ER-positive, locally advanced or metastatic breast cancer (mBC).
AstraZeneca’s Tremelimumab is approved by EMA for non-small cell lung cancer (NSCLC). It is administered through drip in combination with durvalumab and chemotherapy. Its MOA involves blocking CTLA-4 and increasing the number and activity of T-cells.
Curium’s Pylclari is used in adults to detect prostate cancer with a protein PSMA by using a body scan (PET). It is administered through injection before scanning that has API piflufolastat.
Novartis’s Tevimbra is designated as an orphan drug for oesophageal cancer. It is a monoclonal antibody that blocks the PD-1 receptor thus increasing the activity of the immune system.
Neurology
In 2023, EMA approved five drugs in neurology domains UCB’s Zilbrysq is a parental drug used with other medicines in generalised myasthenia gravis. That attaches to the C5 complement protein. Abbvie’s Aquipta is prescribed to adults with migraines who have migraines at least 4 days a month. The API atogepant attaches to receptors that prevent CGRP and amylin-1 protein from binding with them and is given orally.
Neurax Pharma’s Briumvi treats adults with relapsing forms of multiple sclerosis and is administered through drip having API ublituximab which is a Monoclonal Antibody that attaches to CD20 found on the surface of B cells.
Designated as an Orphan drug, Santhera’s Agamree is used for the treatment of Duchenne muscular dystrophy and is given to patients (≥4yrs.). Its MOA involves stopping the production of cytokines which causes inflammation.
The report only covers the highlights from Oncology and Neurology. For a complete report, reach out to us at connect@pharmashots.com
Related Post: A Complete Account of FDA Approvals in 2023

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.














